Carbon 101-part 2 “Carbon Footprint” – here
Carbon 101–part 3 “Drawdown”–here
Combustion

Carbon is a chemical element with the symbol C. Although widely distributed in nature, carbon is not particularly plentiful—it makes up only about 0.025 percent of Earth’s crust—yet it forms more compounds than all the other elements combined. It is also the basic building block for all life on earth.
Oxygen is a chemical element with the symbol O, it is an oxidizing agent that readily forms oxides with most elements as well as with other compounds. For example, rust is oxidized iron. Oxygen is the third-most abundant element in the universe by mass after hydrogen and helium. In the air, two oxygen atoms usually join to make dioxygen or O2, a colourless gas. This gas is often just called oxygen.
The rapid oxidation or burning of carbon produces carbon dioxide (CO2) plus energy in the form of heat and light.
C + O2 → CO2 + Energy
Methane is a chemical compound composed of hydrogen (H) and carbon (C) with the chemical formula CH4. It is the main constituent of natural gas. It is the simplest of the hydrocarbons.
When methane is burned it produces carbon dioxide (CO2), water (H2O) and again energy in the form of heat and light.
CH4 + 2O2 → CO2 + 2H2O + Energy
There are many other hydrocarbons in the mixes which make up natural gas and petroleum, also known as fossil fuels. When they are burned to produce energy, CO2 is always produced.
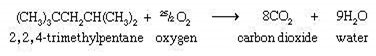
This example is an important component of gasoline.
Hydrocarbons are never found in a pure state and even after refining contain many impurities. These impurities, along with the fact that the combustion of these fuels is never complete, contribute to the air pollution which results from the burning of fossil fuels. These air pollutants, which include sulfur dioxide, nitrogen oxides, and various unburned hydrocarbons are a whole separate issue apart from the release of CO2.
Carbon Dioxide
At room temperature and pressure carbon dioxide (CO2) is a colourless gas with a faint sharp odour and a sour taste. It is heavier than air, when at the same temperature, but rises into the atmosphere in the mix of hot air and gases that result from burning of fossil-fuels.
Human activities—especially fossil-fuel combustion since the Industrial Revolution—are responsible for steady increases in atmospheric concentrations of carbon dioxide, measured in parts per million (ppm).

Once it is added to the atmosphere, it hangs around, for a long time – between 300 and 1,000 years. Thus, as humans change the atmosphere by emitting carbon dioxide, those changes will endure on the timescale of many human lives.
The concentration of carbon dioxide in Earth’s atmosphere on Feb. 8, 2021 was 416.02 ppm. (Find out what the CO2 concentration is today here.) This represents a 48.5 percent increase since the beginning of the Industrial Age, when the concentration was near 280 ppm, and a 16 percent increase since 2000, when it was near 370 ppm.
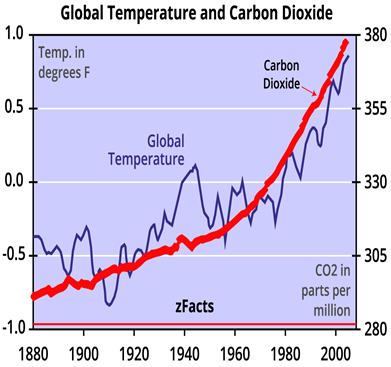
The presence of CO2 in the atmosphere helps keep some of the radiant energy received by Earth from the sun from being returned to space, thus producing the greenhouse effect.
Without CO2 and other greenhouse gases, the average temperature at Earth’s surface would be about −18 °C, rather than the present average of 15 °C.
The problem is that as the concentration of CO2 in the atmosphere rises so does global temperature.
Scientists, climate experts, and governments officials agree that 350 ppm is the “safe” level of carbon dioxide. (hence the climate change activists group name 350.org) This is the level where temperatures would stabilize at 1°C above pre-industrial levels and avoid runaway climate destabilization.
We have already blown past the agreed “safe” level of 350 ppm CO2 and the 1°C of temperature rise. Without massive reductions in CO2 emissions in the coming decades, it is estimated the global average temperature rise will be between 4 and 6°C.
The bottom line is we need to stop emissions and begin to draw down CO2 or face ever increasing temperatures. We are just seeing the beginning of the resulting climate change — more severe weather resulting in more severe floods, droughts, fires, rising oceans, melting glaciers…
Carbon 101-part 2 “Carbon Footprint” – here
Carbon 101–part 3 “Drawdown”–here